Osteoporosis (OP) is a systemic metabolic bone disease characterized by the loss of bone mass and density, which makes bones brittle and prone to fractures. The World Health Organization has recognized it as the second leading cause of risk to human health after cardiovascular disease, earning it the nickname "silent killer." Indeed, OP afflicts millions of people globally. According to the International Osteoporosis Foundation, one in every three women will develop osteoporotic fractures by age 50, as will one in every five men. Those with low calcium intake are more likely to develop OP, particularly postmenopausal women and the elderly.
Many factors play a role in the development of osteoporosis, including genetics, hormones, nutrition, age, and lifestyle. There are usually no indications of bone loss in the early stages. But, once bones are compromised by osteoporosis, symptoms such as back pain caused by a broken or collapsed vertebra, loss of height over time, a stooped posture, and a bone that breaks much more easily than expected may develop.
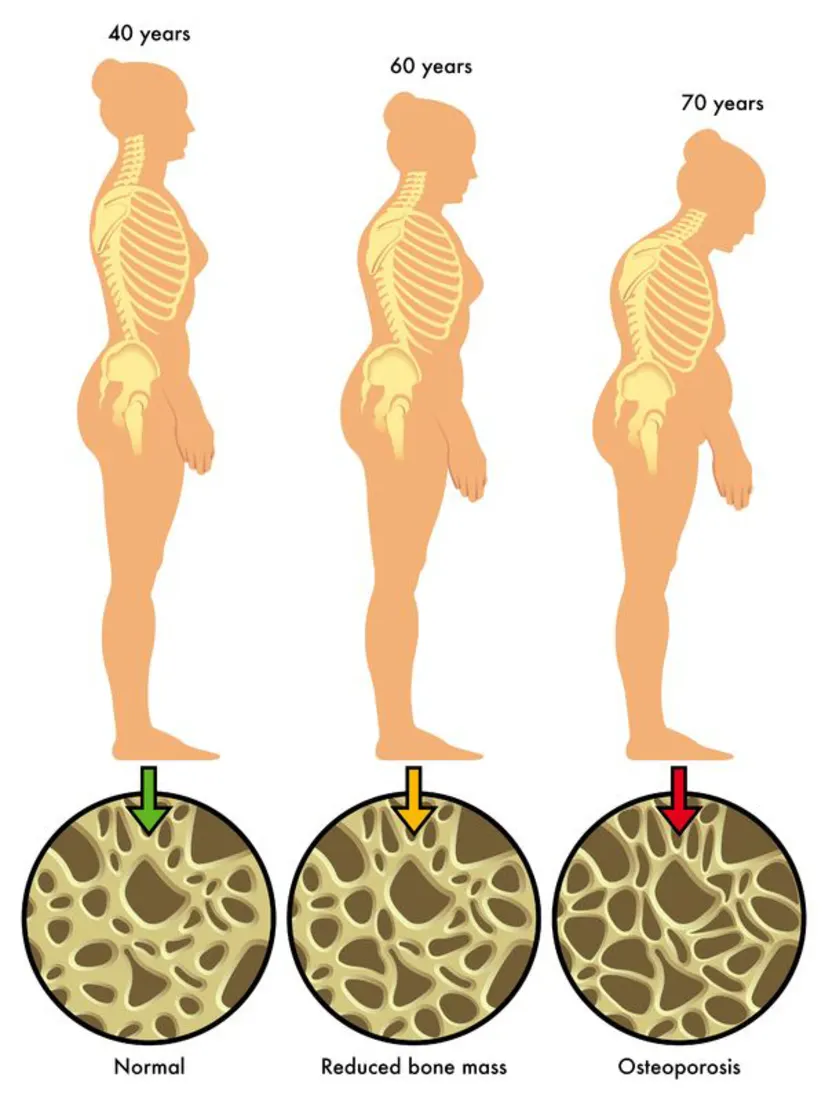
Development of Osteoporosis[1]
Researchers employ animal models, mainly mice, to examine the underlying mechanisms of osteoporosis in order to better understand the disease and discover effective treatments. The selection of animal models for pharmacological efficacy research varies due to the complexity of the pathophysiology and mechanisms of action of potential therapy. An ideal osteoporosis model should satisfy the following criteria: simplicity, economy, and similarity to the human disease. Mouse models are good candidates because they have many similarities to humans in terms of biology and physiology, including bone composition and metabolism.
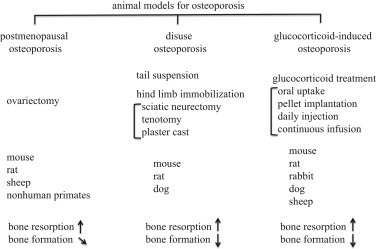
Animal models for osteoporosis[2]
Constructing Mouse Models for Studying Osteoporosis
There are several approaches for constructing osteoporosis mouse models, each with its own set of advantages and drawbacks. The most common strategy includes inducing bone loss through hormonal or dietary manipulation, such as estrogen deficiency or low-calcium diets, which mimics the natural progression of OP. Another method is to introduce genetic mutations that affect bone metabolism, such as knockouts of genes encoding specific proteins involved in bone formation and resorption. This enables researchers to investigate the disease's particular mechanisms and identify prospective therapeutic targets. OP mouse models can also be produced by irradiation, immobilization, and mechanical loading, all of which affect bone metabolism and function.
GemPharmatech’s Osteoporosis Mouse Models
For many years, GemPharmatech has made significant contributions to the animal modeling field, with extensive experience in modeling techniques and model construction. To aid in the development of OP-related drugs, GemPharmatech has developed several types of OP models using both gene editing and surgical approaches to satisfy the need to preclinical evaluation of various types of pharmaceuticals.
Spontaneous OP model
The relationship between osteoblast bone formation and osteoclast bone resorption is essential for maintaining a healthy bone metabolism, as human bone tissue is constantly undergoing reconstruction. The osteoprotegerin (OPG)/receptor activator of nuclear factor-κB (RANK)/receptor activator of nuclear factor-κB ligand (RANKL) signaling pathway connects osteoblasts and osteoclasts. RANKL (TNFSF11) is a member of the TNF superfamily that is involved in immune modulation and bone metabolism (formation/resorption) and is a key activation factor for osteoclast differentiation and maturation. Through its binding to RANK, RANKL regulates bone formation and plays a crucial role in bone reconstruction. OPG binds to RANKL, blocking RANK binding to RANKL and so inhibiting bone resorption and maintaining bone metabolic balance[3]. As a result of the overactivated osteoclasts, overexpression of RANKL or downregulation of OPG levels may result in OP.
GemPharmatech used BAC transgenic technique to create the B6-hRANKL model, which contains the entire human RANKL gene and is able to spontaneously develop osteoporosis symptoms. Moreover, a B6-hRANKL-mOPG+/- model has been generated by crossing B6-hRANKL mice with mOPG-KO mice. Overexpression of RANKL and downregulation of OPG in this model results in a more severe osteoporotic phenotype, which can be used to evaluate the therapeutic efficacy of anti-osteoporotic medicines targeting hRANKL.
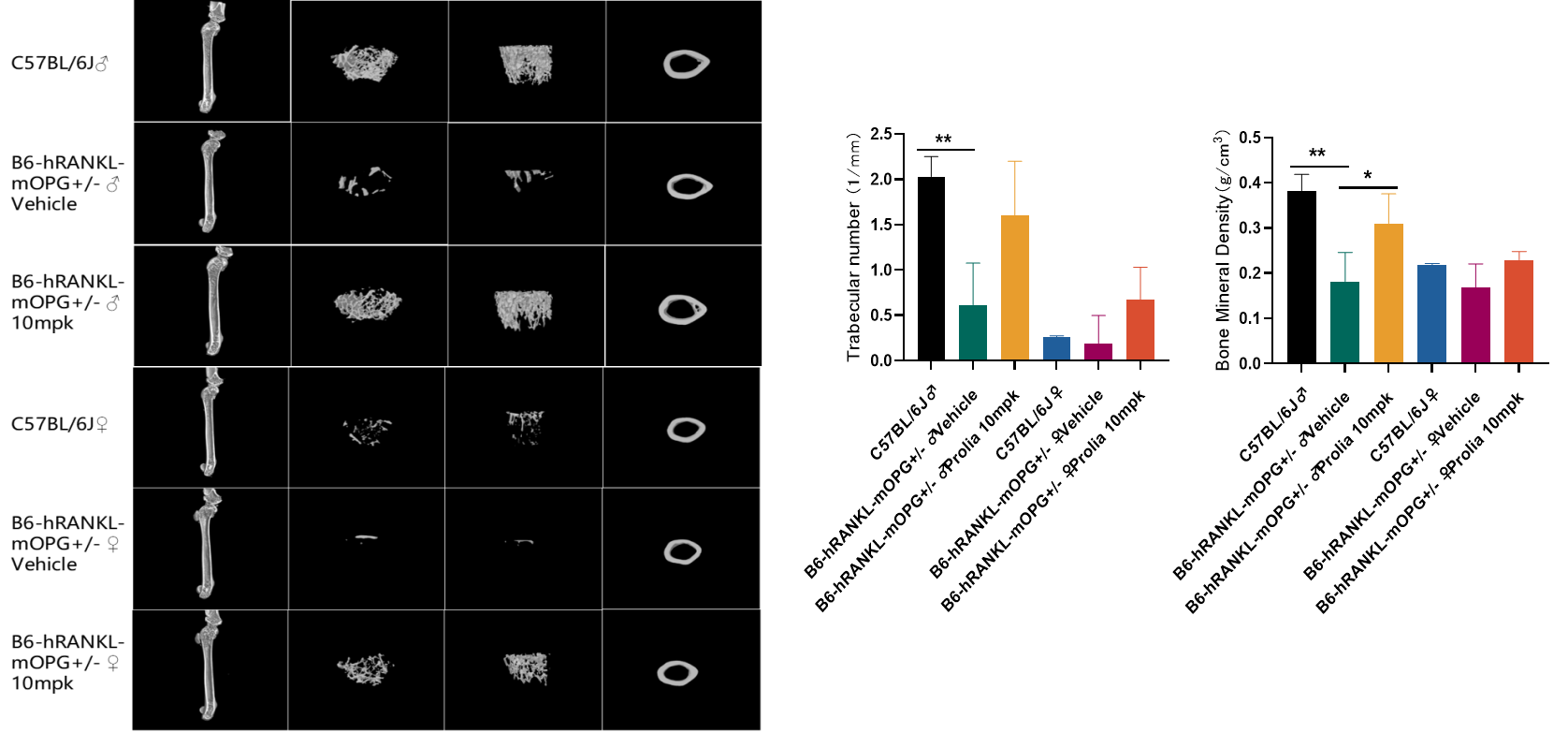
In vivo drug efficacy evaluation using B6-hRANKL-mOPG+/- mice
The micro CT imaging results above indicate that compared to wild-type mice, B6-hRANKL-mOPG+/- mice exhibit reduced number of trabeculae and bone density. However, after intervention with the human RANKL antibody (Prolia or Denosumab), the number of trabeculae and bone density in B6-hRANKL-mOPG+/- mice increased, resulting in a significant improvement in their phenotype.
Ovariectomy (OVX) induced OP model
Estrogen is one of the important substances for maintaining the balance of bone metabolism. It can directly act on osteoclasts through the osteoprotegerin (OPG) / nuclear factor κβ receptor activating factor (RANK) / receptor activator of nuclear factor κβ ligand (RANKL) pathway, promoting osteoclast apoptosis and inhibiting osteoclast differentiation and maturation, thereby reducing bone resorption. Therefore, estrogen deficiency will cause an imbalance in bone metabolism, leading to bone loss or osteoporosis. Ovariectomy can reduce estrogen levels in female mice and simulate postmenopausal osteoporosis in women. GemPharmatech has established an osteoporosis model by removing the ovaries of female C57BL/6JGpt mice.
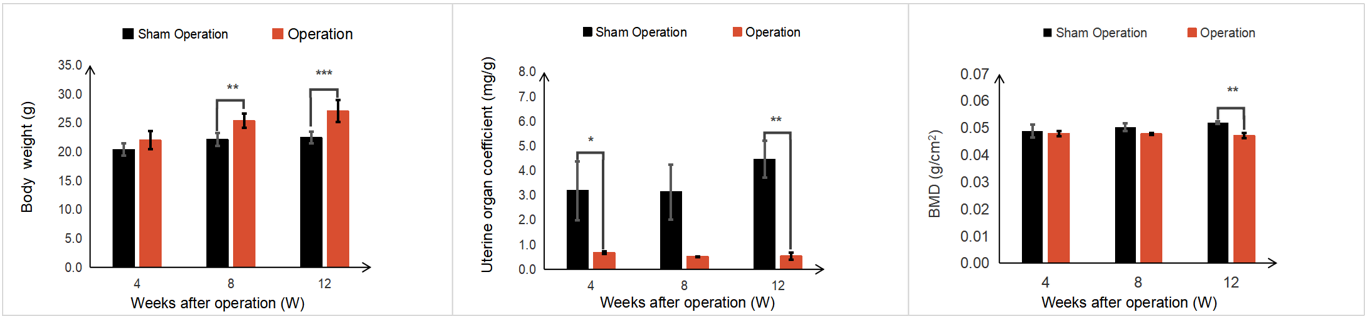
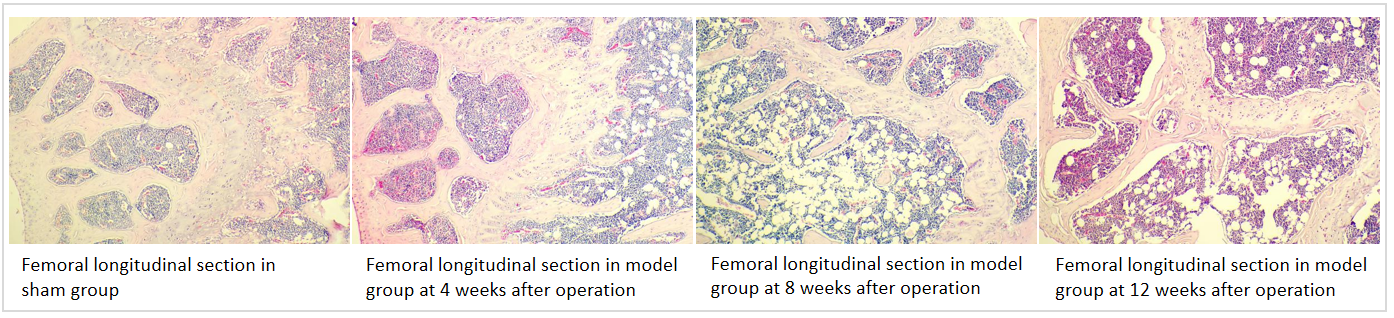
Phenotypic observation of OVX induced OP mice
12 weeks after ovariectomy, the body weight of female mice had increased significantly compared with the sham group. After ovariectomy, the organ coefficient of the uterus and bone mineral density (BMD) of the mice were reduced significantly. H&E staining of mouse femurs showed that the model group exhibited rough bone trabecular edges, increased bone trabecular space, irregular arrangement of bone trabeculae with breakpoints, and increased vacuoles in the bone marrow cavity compared with the sham group.
OP models comparison
OVX induced Model | B6-hRANKL-Tg | B6-hRANKL-mOPG+/- | |
Induction mechanism/mutation mechanism
| Estrogen plays an important role in regulating bone metabolism and maintaining the stability of the internal environment. Because estrogen levels decrease after ovariectomy, osteoclasts exceed osteoblasts in both number and activity and osteoclasts enhance bone resorption, leading to OP.
| RANKL activates osteoclast differentiation and promotes bone resorption through binding to receptor RANK. Overexpression of RANKL can lead to excessive activation of osteoclasts and the occurrence of OP. | Osteoprotegerin (OPG) competitively binds to RANKL with RANK, thereby inhibiting the formation and activation of osteoclasts. Overexpression of RANKL and knockout of OPG can lead to excessive differentiation of osteoclasts and the occurrence of OP.
|
Phenotypic characteristics | OVX-induced mice exhibit a phenotype of increased body weight, decreased uterine organ coefficient, decreased bone density, increased bone trabecular space, and irregular arrangement of bone trabeculae with breakpoints. | The bone density, bone trabecular volume fraction, and bone trabecular number decrease and bone trabecular separation increase in B6-hRANKL-Tg mice. | The number of bone trabeculae and bone mineral density significantly decrease and serum alkaline phosphatase (AKP) significantly increase in B6-hRANKL-mOPG+/- mice. The phenotype of B6-hRANKL-mOPG+/- mice is more severe than B6-hRANKL-Tg mice. |
Application | This model simulates osteoporosis symptoms caused by estrogen deficiency in adult women and can be used for small molecule drug interventions (estrogen, vitamin D analogues, etc.). | This model can be used to evaluate antibody drugs targeting human RANKL (eg: Denosumab) and bone protective polypeptides. | This model can be used to evaluate antibody drugs targeting human RANKL, bone protective polypeptides, or a combination of these. |
References:
https://www.healthcentral.com/condition/osteoporosis/types-osteoporosis-primary-or-secondary
Komori T. Animal models for osteoporosis. Eur J Pharmacol. 2015 Jul 15;759:287-94. doi: 10.1016/j.ejphar.2015.03.028. Epub 2015 Mar 24.
Rinotas, Vagelis, et al. Novel genetic models of osteoporosis by overexpression of human RANKL in transgenic mice. Journal of Bone and Mineral Research 29.5 (2014): 1158-1169.
https://www.osteoporosis.foundation/health-professionals/about-osteoporosis/epidemiology
https://www.mayoclinic.org/diseases-conditions/osteoporosis/symptoms-causes/syc-20351968
Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. American journal of obstetrics and gynecology. 2006 Jun 1;194(6):S3-11.
Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. Journal of gerontology. 2013 Jul 1;68(7):771-8.
Wong IP, Latchford KJ. The role of mouse models in elucidating the mechanisms of action of bisphosphonates. BoneKEy reports. 2015 Jan 28;4.
Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy reports. 2014 Oct 22;3.


