Atopic dermatitis (AD), also known as eczema, is a chronic inflammatory skin disease that affects millions of people worldwide. It is characterized by itching, redness, dryness, and scaling of the skin, which can lead to significant discomfort and impaired quality of life. Atopic dermatitis is a complex condition with a multifactorial pathogenesis that involves interactions between genetic, environmental, and immunological factors. Despite extensive research, the underlying mechanisms of AD are not fully understood, and current treatment options are often inadequate.
Atopic dermatitis was first formally described and defined in the 1930s, with the word "atopic" originated from the Greek word "atopos", meaning "without place, inexplicable". The disease is now understood as "genetically related hypersensitivity to environmental variables" [1]. AD often begins in childhood, but can recur until adulthood or appear for the first time at any age. The disease can run indefinitely or in a "flare-relapse" pattern[2]. Early onset, family history, and early exposure to allergens are all risk factors for AD.
According to the Global Burden of Disease (GBD) study, the global incidence of atopic dermatitis has been increasing year over year for the last 30 years (1987-2017), with developed countries accounting for up to one-fifth of atopic dermatitis sufferers[1]. According to two studies conducted in the United States, the total direct and indirect expenses of AD for children and adults in 2013 were as high as $3,300 per person per year. The number of atopic dermatitis patients has increased in recent years, and atopic dermatitis has evolved into a prevalent and refractory skin disease in dermatology.
A fundamental feature of AD onset is the change of the epidermal barrier and the invasion of external antigens[4]. The skin acts as the first line of defense for the human body, efficiently resisting physical, chemical, microbiological, and other assaults. According to research on atopic dermatitis susceptibility, the loss of the epidermal barrier can be induced by a range of variables, including environmental temperature and humidity, allergens, physical and chemical stimulation, scratching, and FLG (filaggrin) gene mutations[5]. Microbial dysbiosis can wreak havoc on the barrier, with Staphylococcus aureus and Malassezia being the major causes[3, 4].
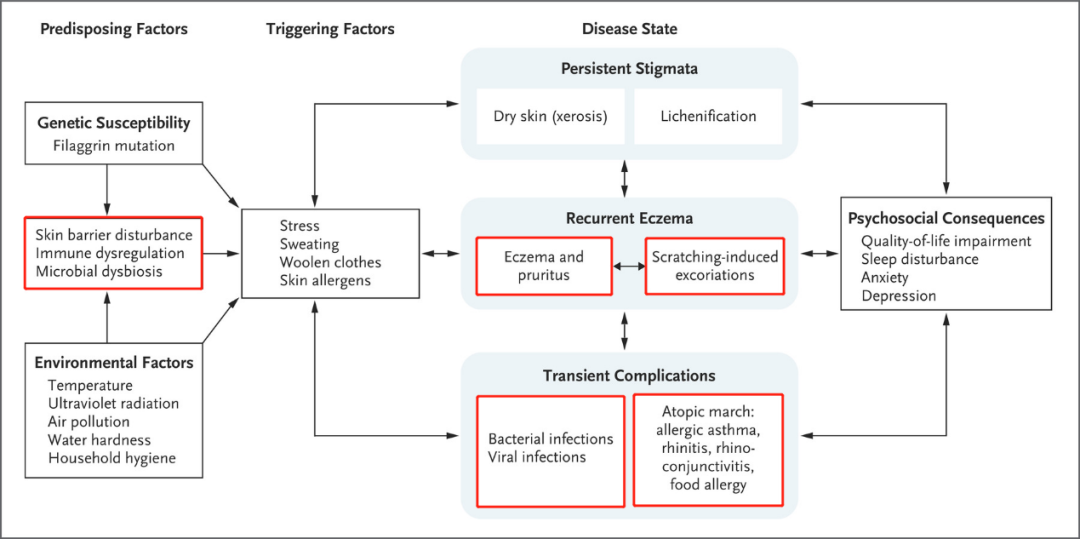
AD pathogenesis, progression and consequences[3]
The exact pathogenic mechanism of atopic dermatitis is still not completely understood. However, the most widely accepted theory is the "atopic march," which suggests that atopic dermatitis is the first manifestation of a series of allergic diseases that can develop in a sequence, including asthma and allergic rhinitis. The collapse of the epidermal barrier is the first phase in the development of AD, with the disordered immune system also being a key aspect. The subcutaneous immune system is activated by alterations in skin function and the invasion of external antigens caused by various combinations of both genetic and environmental factors. Generally, the innate immune system, which includes dendritic cells, macrophages, neutrophils, and other immune cells, is activated first. When the subcutaneous immune system is repeatedly activated, persistent and chronic inflammation develops, which is associated with the activation of adaptive immunity. The activation of Th2 cells and related inflammatory factors is thought to be the primary pathway leading to the inflammatory phenotype of the AD. Activated Th2 cells release cytokines into the skin, mainly IL-4, IL-13, and IL-31, which activate B and plasma cells, promoting inflammation and the production of antigen-specific IgE, decreasing the barrier function, and causing itching[6, 7].
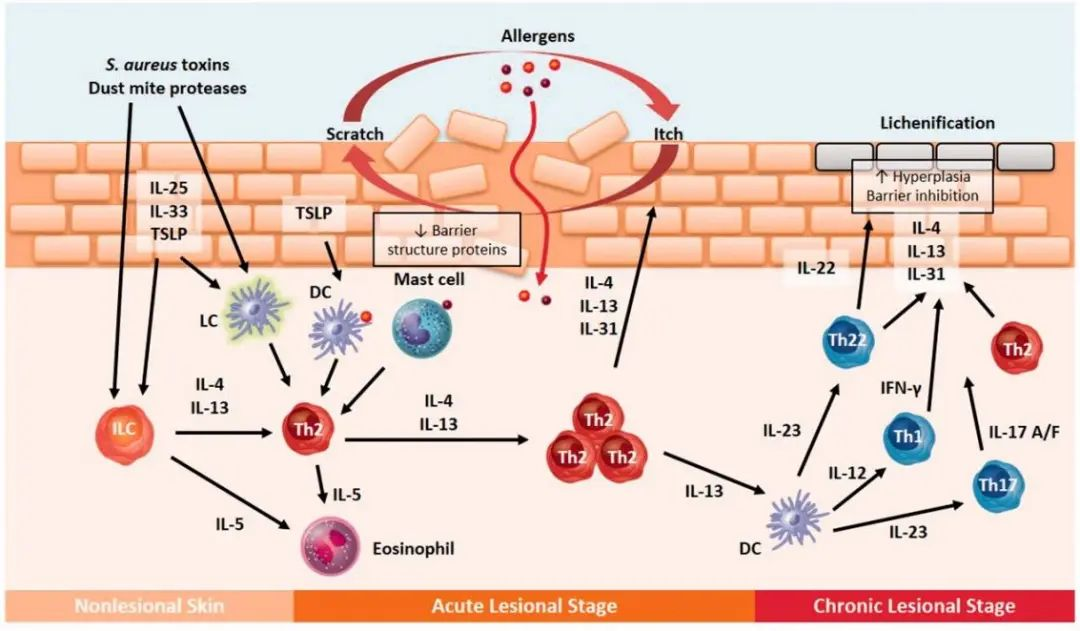
Skin barrier dysfunction and immune response in AD[7]
In clinical practice, the purpose of treating AD is to improve the patient's quality of life by alleviating clinical symptoms, eliminating triggering factors and preventing recurrence. However, achieving complete elimination of AD and avoiding recurrence is often difficult to accomplish. Controlling skin lesions and suppressing excessive activation of the immune system are the principles. The 2020 revised consensus on AD treatment points out that topical corticosteroids (TCS) are still the first-line therapy for AD.
Common treatment options include[8]:
1. use of moisturizers and avoidance of triggering factors (non-specific factors, allergens, etc.);
2. sysmatic treatment with topical corticosteroids (TCS) and calcineurin inhibitors (TCI) according to the type and location of skin lesions;
3. oral antihistamines for the treatment of coexisting allergic conditions (urticaria, allergic rhinitis) or pruritus;
4. sysmatic treatment of antimicrobial infections;
5. immunosuppressive agents (such as cyclosporine and methotrexate) for severe AD;
6. emerging targeted therapies (such as dupilumab).
It should be noted that currently there is no specific drug for AD treatment. Existing AD treatments are mostly broad-spectrum drugs, and their widespread or long-term use often leads to various side effects, such as skin atrophy, immune system disorders, and abnormal growth and development[8, 9].
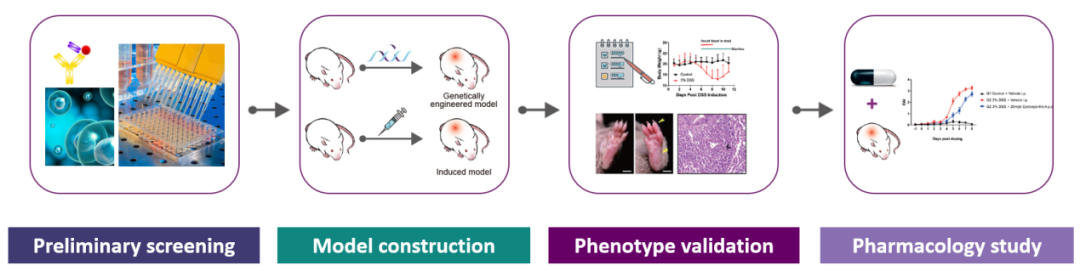
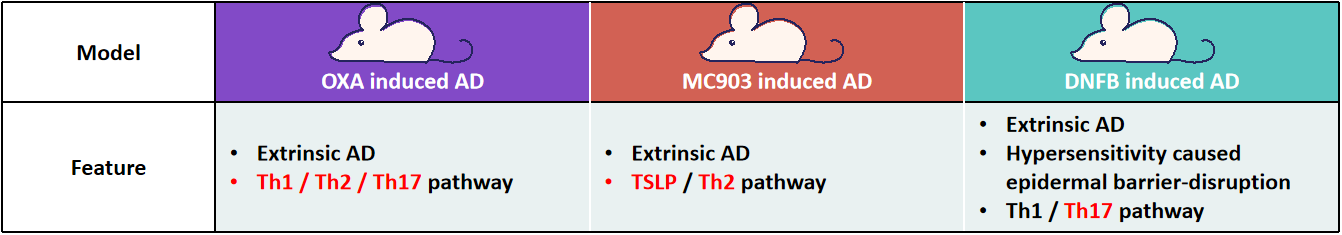
With the meticulous exploration of the pathological mechanisms of AD, various molecular basis for AD have gradually been systematically elucidated. Among these, the discovery of targets for AD therapies (such as those targeting barrier dysfunction, pruritic pathways, specific immune signaling molecules, and familial genotypes) can pave the way for a promising new era of individualized treatments[6, 10-11]. Reliable and well-characterized mouse models are essential for translational research and drug development. GemPharmatech has successfully built several AD mouse models and offers preclinical services for AD drug evaluation to promote innovation and the development of AD medications.
GPT’s preclinical services and model resources for AD
Reference:
1. Weidinger, S. and N. Novak, Atopic dermatitis. The Lancet, 2016. 387(10023): p. 1109-1122.
2. Garmhausen, D., et al., Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy, 2013. 68(4): p. 498-506.
3. Ständer, S., Atopic Dermatitis. The New England journal of medicine. 384(12): p. 1136-1143.
4. Sroka-Tomaszewska, J. and M. Trzeciak, Molecular Mechanisms of Atopic Dermatitis Pathogenesis. International Journal of Molecular Sciences, 2021. 22(8): p. 4130.
5. Cork, M.J., et al., Epidermal Barrier Dysfunction in Atopic Dermatitis. Journal of Investigative Dermatology, 2009. 129(8): p. 1892-1908.
6. Nutten, S., Atopic dermatitis: global epidemiology and risk factors. Annals of nutrition & metabolism. 66 Suppl 1: p. 8-16.
7. Cork, M.J., S.G. Danby, and G.S. Ogg, Atopic dermatitis epidemiology and unmet need in the United Kingdom. The Journal of dermatological treatment. 31(8): p. 801-809.
8. Simon, D. and T. Bieber, Systemic therapy for atopic dermatitis. Allergy. 69(1): p. 46-55.
9. Hijnen, D.J., et al., Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol, 2007. 21(1): p. 85-9.
10. Bieber, T., Atopic dermatitis: an expanding therapeutic pipeline for a complex dis ease. Nature reviews. Drug discovery.21(1): p. 21-40.
11. Brunner, P.M., E. Guttman-Yassky, and D.Y.M. Leung, The immunology of atopic dermatitis and its reversibility with broad-s pectrum and targeted therapies. The Journal of allergy and clinical immunology. 139(4S): p. S65-S76.


